Tau Protein in Alzheimer’s Disease:
Tau protein is a crucial player in the structure and function of neurons, helping maintain cellular integrity and enabling efficient communication across cells. In Alzheimer’s disease, however, tau’s role changes, contributing to neurodegeneration and cognitive decline. This blog delves into tau protein’s function, its role in Alzheimer’s pathology, and the latest research on tau-targeted therapies.
Understanding Tau Protein and Its Role in Healthy Neurons
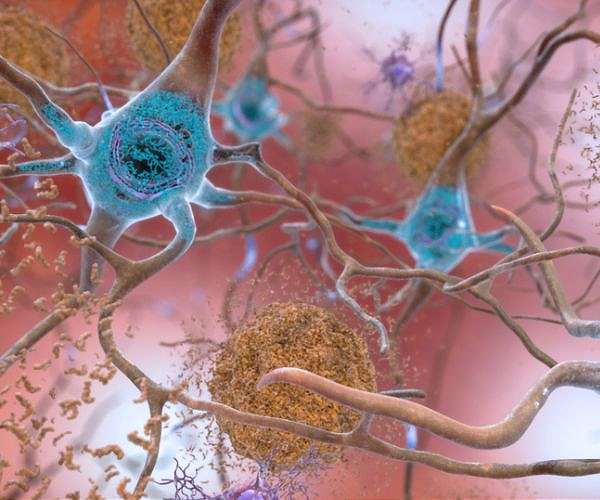
Tau protein is a microtubule-associated protein found mainly in neurons, where it is essential for stabilizing microtubules—cellular structures involved in maintaining the shape and transport functions of neurons. Microtubules help create a “skeleton” that not only supports the cell’s structure but also facilitates the transport of molecules along axons, the elongated structures that neurons use to communicate with each other. Proper microtubule stability is essential for the function and survival of neurons, especially given their dependence on long-distance molecular transport in the brain .
Tau protein works to bind microtubules, providing stability and organizing the structure of neurons. However, tau’s function is tightly regulated by phosphorylation—the addition of phosphate groups. Under normal conditions, tau phosphorylation is balanced, allowing it to bind microtubules without causing harm. When this balance is disrupted, tau proteins can become “hyperphosphorylated,” a key step in the development of Alzheimer’s disease .
Pathology of Tau in Alzheimer’s Disease
Tau tangles originate in the brainstem, a vital structure that serves as the crucial link between the brain and the spinal cord. From the brainstem, these abnormal protein accumulations begin their journey upward, targeting two essential regions of the brain significantly associated with memory function. The first destination is the entorhinal cortex, often referred to as the gateway to the brain’s learning center. This area is particularly susceptible, with some of the earliest damage in Alzheimer’s disease manifesting in its neurons. Following this initial impact, the tau tangles advance into the hippocampus, the brain’s primary hub for processing new information. Here, the brain works to understand novel concepts—like mastering the rules of a board game—and forms short-term memories. The buildup of tau tangles in these critical regions is just one of many biological changes associated with Alzheimer’s disease, contributing to the hallmark symptoms of memory loss and cognitive decline. The presence of abnormal tau proteins in the brain correlates closely with the stage and severity of the disease, making the tracking of tau levels a valuable tool for predicting and identifying cognitive deterioration at earlier stages.
Learn more in the video below.
Watch: How Alzheimer’s Changes the Brain
Tau Protein vs. Amyloid-Beta in Alzheimer’s Disease
While amyloid-beta plaques have long been considered the primary markers of Alzheimer’s, tau tangles have increasingly gained attention for their direct role in neuronal damage. Unlike amyloid-beta, which forms extracellular plaques, tau tangles develop within neurons, leading to cell death and the loss of neural connections, particularly in memory-related regions like the hippocampus. Research has shown that tau pathology correlates more closely with cognitive decline in Alzheimer’s than amyloid-beta, suggesting that tau might be a more direct target for therapeutic intervention .
The spread of tau pathology follows a predictable pattern, beginning in areas crucial for memory formation and navigation, such as the entorhinal cortex and hippocampus. As the disease progresses, tau tangles spread to the neocortex, leading to impairments in cognitive functions like reasoning and language. This spread, known as Braak staging, is used to assess the severity and progression of Alzheimer’s pathology .
Current Research on Tau-Targeted Therapies
With tau’s essential role in Alzheimer’s pathology becoming clearer, there is growing interest in tau-targeted therapies. The primary aim of these therapies is to prevent tau hyperphosphorylation, reduce tau aggregation, and remove existing tangles. Here’s a look at some of the most promising approaches:
1. Tau Protein Inhibitors
Tau protein inhibitors aim to block the formation of tau tangles by stabilizing the protein and preventing hyperphosphorylation. For example, kinase inhibitors are drugs designed to inhibit enzymes responsible for adding phosphate groups to tau proteins. By targeting these kinases, researchers hope to reduce tau phosphorylation levels, stopping or slowing the formation of neurofibrillary tangles .
Researchers are also exploring small molecules that can directly bind to tau, stabilizing it and preventing it from detaching from microtubules. Early-stage clinical trials have shown some promise, but these approaches remain in development, as challenges exist in targeting tau without interfering with normal cellular processes .
2. Monoclonal Antibodies
Monoclonal antibodies, which have shown success in targeting amyloid-beta, are now being adapted to target tau. These antibodies can recognize and bind specifically to pathological forms of tau, marking them for clearance by the immune system. One such therapy, semorinemab, has shown initial promise in reducing tau accumulation and slowing cognitive decline in Alzheimer’s patients .
Monoclonal antibodies targeting tau are still in clinical trials, but preliminary results indicate that they can reduce the spread of tau tangles. While these therapies are in their infancy compared to amyloid-targeted treatments, the specificity of monoclonal antibodies for abnormal tau structures offers hope for minimizing adverse effects .
3. Gene Therapy
Gene therapy presents another approach to treating tau pathology. Using gene-editing technologies like CRISPR, scientists aim to alter or “silence” genes responsible for tau production, reducing tau levels in the brain. By directly targeting the genes that drive tau synthesis or those that regulate its phosphorylation, researchers hope to prevent tau buildup entirely. However, gene therapy is still at an experimental stage, and more research is needed to ensure its safety and efficacy in human patients .
4. RNA-Based Therapies
RNA-based therapies, such as RNA interference (RNAi) and antisense oligonucleotides (ASOs), offer another approach by targeting tau production at the RNA level. By binding to specific RNA sequences, these therapies prevent the translation of tau protein, effectively reducing its presence within cells. Studies using ASOs in animal models have shown promising reductions in tau pathology, though human trials are still underway .
Tau-Targeted Therapies: Challenges and Future Directions
Tau-targeted therapies represent a promising frontier in Alzheimer’s treatment, but challenges remain. One issue is the need for early intervention; by the time tau tangles form, significant damage has already occurred. Detecting Alzheimer’s at a stage when tau-targeted treatments can be most effective is therefore essential. Biomarkers in cerebrospinal fluid and advanced imaging techniques are currently being developed to help identify tau pathology earlier in the disease process .
Another challenge lies in tau’s role beyond Alzheimer’s, as abnormal tau aggregates are implicated in several other neurodegenerative diseases, such as frontotemporal dementia and progressive supranuclear palsy. Understanding the unique and shared mechanisms of tau pathology in these conditions may provide insights that benefit broader therapeutic applications .
Conclusion: The Future of Tau-Targeted Alzheimer’s Treatments
While amyloid-beta has historically dominated Alzheimer’s research, tau protein has emerged as a critical component in understanding the disease’s progression. Tau pathology’s direct impact on neurons, combined with its close association with cognitive decline, underscores its significance as a therapeutic target. Although tau-targeted treatments are still in early stages, the advancements made thus far show promise. With ongoing research, we may soon have effective strategies to intervene against tau pathology, offering hope for Alzheimer’s patients and their families.
Continued advancements in biotechnology, gene editing, and immunotherapy, along with a deeper understanding of tau’s role, bring us closer to effective interventions. As researchers refine these therapies and push through clinical trials, there is optimism that tau-targeted treatments may soon become a core component of Alzheimer’s care, changing the trajectory of this devastating disease and improving quality of life for those affected.
References
- “The Role of Tau in Neurodegeneration.” Journal of Neuroscience, 2018.
- Selkoe, D. J., & Hardy, J. (2016). “The amyloid hypothesis of Alzheimer’s disease at 25 years.” EMBO Molecular Medicine.
- Arendt, T. et al. “Tau and neurodegeneration: How tau pathology spreads in Alzheimer’s disease.” Acta Neuropathologica, 2016.
- “Pathology of tau protein in Alzheimer’s Disease.” Frontiers in Neuroscience, 2020.
- Braak, H. & Braak, E. “Staging of Alzheimer-related cortical destruction.” Neurobiology of Aging, 1991.
- Götz, J. et al. “The role of tau protein in Alzheimer’s disease.” Biochimica et Biophysica Acta, 2013.
- Morris, M. et al. “Tau and amyloid-β pathology in Alzheimer’s disease.” Nature Reviews Neuroscience, 2018.
- “Tau Inhibitors and Alzheimer’s Treatment.” Alzheimer’s Research Journal, 2019.
- Kovacs, G. G. et al. “Tau Pathology in Neurodegenerative Diseases.” Brain Pathology, 2021.
- “Monoclonal Antibodies for Alzheimer’s.” Journal of Clinical Neurology, 2022.
- Long, J. M. et al. “Alzheimer Disease – An Update on Pathobiology and Treatment Strategies.” Cell, 2019.
- “Gene Therapy in Alzheimer’s Research.” Cellular and Molecular Neuroscience, 2020.
- “RNA-Based Treatments for Alzheimer’s.” Experimental Neurology, 2021.
- “Advances in Alzheimer’s Biomarkers.” Lancet Neurology, 2019.
- Spillantini, M. G., & Goedert, M. “Tau protein pathology in neurodegenerative diseases.” Science, 2013.
![]()
Discover more from Shaivam Kale
Subscribe to get the latest posts to your email.